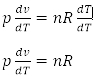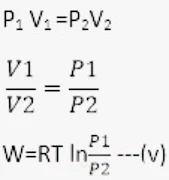Unit-2 Heat and Thermodynamics
PDF Link:- View
Unit-2 Heat and Thermodynamics
Thermodynamics System
a) Open system
b) Closed System
c) Isolated System
Thermodynamics Process
1) Isothermal Process
2) Isochoric process
3) Isobaric process
The process in which pressure remains constant is called the isobaric process.
4) Adiabatic process
The process in which pressure, volume, and temperature change but the amount of heat remain constant is called the adiabatic process.
Work done by expansion

Fig: Work done by expansion
Let us consider a gas kept inside a cylinder provided with a movable and frictionless piston at pressure 'p', volume 'v', and temperature 'T'. Suppose the gas expands by a small volume 'dv' and the piston moved forward by distance 'dx'. If A is the cross-sectional area of the cylinder then force on the piston,
F = P.A [∴ P=F/A]
Also, the change in volume
dv = Adx
Now,
dw= F.dx
dw= PAdx
dw= Pdv
which is external work done
PV diagram
Internal energy
For Ideal gas, Internal energy = kinetic energy
First Law of thermodynamics
It states that if a certain amount of heat is supplied to the given system then a part of the heat may be used to increase the internal energy and the remaining part is used for work done.
If dQ is the amount of heat supplied to the system and du, dw is the increases in internal energy and external work done respectively, then according to the first law of thermodynamics.
dQ = du + dw --- (i)
since
dQ = du + pdv
If dv = 0 then dw = 0
∴ dQ = du
It means the total amount of heat supplied is used to increase external work done only when the volume is constant.
Heat Capacity of gas
Molar heat as constant volume (Cv)
It is defined as the amount of heat required to rise the temperature of 1 mole of gas through 1 kelvin at constant volume. It is denoted by Cv.
Molar heat as constant pressure (Cp)
It is defined as the amount of heat required to rise the temperature of 1 mole of gas through 1 kelvin at constant pressure. It is denoted by Cp.
Mayer's formula (Cp-Cv=R)
Let us consider 'n' mole of gas kept in a cylinder provided with the movable and frictionless piston at pressure 'P', volume 'V', and Temperature 'T'. Suppose the gas is heated at constant volume initially and dQ is the amount of heat given to the system to increase its temperature by dT then,
dQ = nCvdT --- (i)
From the first law of thermodynamics,
dQ = du + PdV
Since, at constant volume, dv=0
so, dQ = du
∴ du = nCv dT --- (ii)
Again the gas is heated at constant pressure and dQ be the amount of heat given to the system to increase the temperature by dT.
dQ = nCpdT --- (iii)
Again from first law of thermodynamics
dQ = du + Pdv
nCpdT = nCvdT + Pdv---(iv) ∴using equation (ii)
From idea gas equation
PV = nRT
Differentiating both sides with respect to at T at constant pressure.
Equation of Adiabatic Process
From the first law of thermodynamics
dQ = du + pdv --- (i)
For adiabatic process
dQ = 0 (∴ heat constant)
du + Pdv = 0 --- (ii)
If Cv is the molar heat capacity at a constant volume for one mole of gas,
du = 1Cvdt --- (iii)
using equation (iii) in equation (ii)
CvdT + Pdv = 0 --- (iv)
Also ideal gas equation for 1 mole of gas
PV = RT
Differentiating both sides with respect to 'T'
P dv / dT + V dP / dT = R dT / dT
P dv + vdP = R dT
dT = Pdv + Vdp / R --- (v)
using equation (v) in equation (iv)
Cv ( Pdv + Vdp / R ) + Pdv = 0
Cv (Pdv + Vdp) + RPdv = 0
(CV+R) Pdv + CvVdp = 0 [ ∴ cp-cv=R]
CpPdv + CvVdp = 0
Dividing both side by CvPv
Integrating on both sides
In terms of pressure and volume
In terms of temperature and volume
We have,
In terms of pressure and temperature
Work done by an isothermal process
dw = PdV --- (i)
Thus total work done
for 1 mole of the gas ideal gas equation be,
PV = RT
P = RT / V --- (iii)
using equation (iii) in equation (ii)
Work done by Adiabatic process
For one mole of gas
Equations (iv) and (iii) are required expressions for n mole of gas during the adiabatic Process.